The first atomic theorist was Democritus a Greek scientist and philosopher who lived in the. An atom is the smallest unit of an element that can participate in a chemical change.
Unlike Democritus Dalton based his ideas on experiments.

Summarize the development of atomic theory. Emblematic Images in the Scientific Revolution. His theory helped explain observations that he and other scientists had made about elements and compounds. Bohr proposed that electrons are arranged in concentric circular paths around the nucleus 5.
John Dalton developed the atomic theory around the 1800s. The atomic theory was modified with the discovery of every new element. Thomsons cathode ray tube showed that atoms contain small negatively charged particles called electrons.
He stated 4 postulates. 6 Development of Atomic Theory History Chemists Timeline 1. It wasnt until 1803 that the English chemist John Dalton started to develop a more scientific definition of the atom.
Revolutionary Thinkers from the Scientific Revolution to. So for example sweet things are made of smooth atoms bitter things are made of sharp. All atoms of a given element are identical but differ from those of any other element.
The Early Greeks Theory. How a coherent scientific theory is developed by observation hypothesis and experiment. Matter is composed of exceedingly small particles called atoms.
However Aristotle did not accept the theory and it was ignored for many centuries. Early atomic theory stated that the characteristics of an object are determined by the shape of its atoms. Development of Atomic Theory The atomic theory which holds that matter is composed of tiny indivisible particles in constant motion was proposed in the 5th cent.
The Trial of Galileo. Galileo and the Scientific Method. BC by the Greek philosophers Leucippus and Democritus and was adopted by the Roman Lucretius.
The discovery of new evidence resulted in changes to the atomic theory. Daltons atomic theory also stated that all compounds were composed of combinations of these atoms in defined ratios. The key scientists involved in developing atomic theory.
Development of the Atomic Theory continued What Was the First Scientific Theory of Atoms. Formation of the atomic theory. The atomic theory led to the creation of the law of multiple proportions.
He drew on the ideas of the Ancient Greeks in describing atoms as small hard spheres that are indivisible and that atoms of a given element are identical to each other. Democritus atomic theory posited that all matter is made up small indestructible units he called atoms. First and foremost John Dalton introduced about the concept of atom.
In this lesson we will learn. Here are the postulates of Daltons atomic theory. The Development of Atomic Theory.
Atomic theory originated as a philosophical concept in ancient India. Daltons atomic theory proposed that all matter was composed of atoms indivisible and indestructible building blocks. In 1803 he revived the atomic theory of matter.
Evolution of Atomic Theory Although no one has actually seen the inside of an atom experiments have demonstrated much about atomic structure. While all atoms of an element were identical different elements had atoms of differing size and mass. In modern atomic theory the locations of electrons are not fixed.
Each element is composed of extremely small particles called atoms. It took until the end of the 18th century for science to provide concrete evidence of. From White Light to Rainbow Brite.
A Brief History of Atomic Theory The Atom and Atomism. Advancements in atomic models proved the atomic theory was accurate. Thomson proposed that the atomic model in which negatively charged electrons were embedded in a positively charged mass 3.
Religion and the Scientific Revolution. The steps in the breakthroughs to lead to our modern understanding of the atom. Millikan discovered that there is a fundamental electric chargethe charge of an electron.
Asked by Wiki User. Wiki User Answered 2012-10-19 193821. The first scientific theory about atoms was published by John Dalton in 1803.
Summerize the development of the atomic theory. He developed the atomic theory. 1873 James Clerk Maxwell proposed the theory of electromagnetism and made the connection between light and electromagnetic waves.
First published in 1807 many of Daltons hypotheses about the microscopic features of matter are still valid in modern atomic theory. Which statement best summarizes the development of the atomic theory over time. Law of Multiple Proportions The law of multiple proportions states that if two elements form more than one compound between them the masses of one element combined with a fixed mass of the second element form in ratios of small integers.
Development of atomic theory The concept of the atom that Western scientists accepted in broad outline from the 1600s until about 1900 originated with Greek philosophers in the 5th century bce. Rutherford discovered that the atoms are mainly empty space.
Bitte scrollen Sie nach unten und klicken Sie um jeden von ihnen zu sehen. Note that rounding errors may occur so always check the results.
Entiteta Gooey Smisel Atomic Mass Unit Susiessewingsensations Com
Antonyms for Atomic mass unit amu.
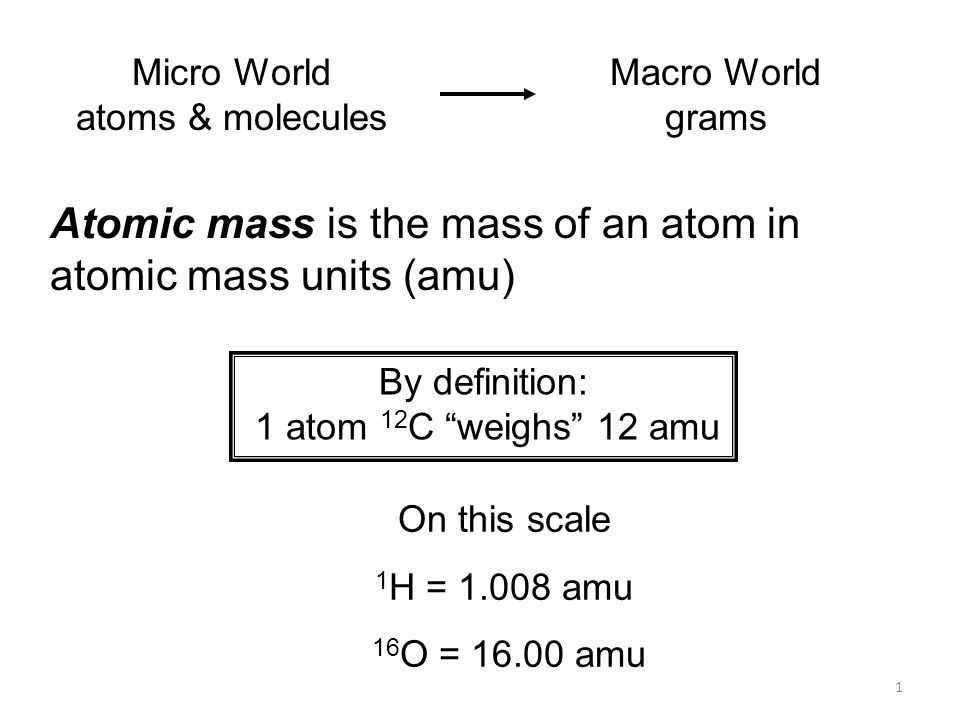
Amu atomic mass unit. One atomic mass unit is defined as exactly of the mass of an atom of the nuclide 12 C the predominant isotope of carbon. Kilotonne tonne. Atomic mass unit to microgram mcg 166110 -18.
1 amu 16610-24gthe average atomic mass for an element is the weighted average of the masses of all the isotopes of an element is correct for atomic mass unit amu. 3 Zeilen 166110 -21. A unit of mass equal to 1 12 the mass of an atom of the most common isotope of carbon carbon 12 which is assigned a mass of 12.
Instead of mmu mDa or and 10 -3 u should be used. The American Heritage Student Science Dictionary Second Edition. Definition von AMU was bedeutet AMU.
The atomic mass unit u or amu is one unit for measuring the atomic mass of particles. Atomic weight is measured in atomic mass units amu also called daltons. A hydrogen atom has a mass of 1 atomic mass unit since its mass is 1 12 the mass of carbon 12.
What are synonyms for Atomic mass unit amu. Andere Bedeutungen von AMU Neben Atomare Masseneinheit hat AMU andere Bedeutungen. The unit also known as the dalton is often abbreviated amu and is designated by the symbol u.
This is equal to 166053892 10 -27 kilograms making it useful for talking about the mass of individual atoms or molecules. Atomic Mass Unit AMU The periodic table of elements contains every atom known to mankind. Atomic mass unit or amu The SI base unit for mass is the kilogram.
Each unique atom has a unique atomic number and atomic mass. It is a unit of mass used to express atomic masses and molecular masses. See below for a list of chemical elements and their atomic weights.
Sie sind auf der linken Seite unten aufgeführt. 1 amu 16610-24gthe average atomic mass for an element is the weighted average of the masses of all the isotopes of an element. It is defined as one-twelfth 112 of the mass of an unbonded Carbon -12.
It is a common mistake to use the deprecated term atomic mass unit and the deprecated unit symbol amu for the unit of mass defined as one-twelfth the mass of single atom 12 C. 1 kilogram is equal to 60229552894949E26 atomic mass unit or 60229552894949E26 amu. An arbitrarily defined unit in terms of which the masses of individual atoms are expressed.
Atomic mass unitu amu the unit of mass equal to ¹₁₂ the mass of the nuclide of carbon 12. The unit symbol mmu meaning a millimass unit is also an appropriate unit in SI. Other articles where Atomic mass unit is discussed.
The atomic number is the number of protons. 1 word related to atomic mass unit. In chemistry an atomic mass unit or AMU is a physical constant equal to one-twelfth of the mass of an unbound atom of carbon -12.
