The elements marked with an asterisk in the 2nd column have no stable nuclides. However there wasnt always a standard method of numbering groups so this can be confusing when consulting older tables.
 How Do You Determine What Group Number An Element Is In Socratic
How Do You Determine What Group Number An Element Is In Socratic
Periods 4 and 5 have 18 elements.
Periodic table group numbers. Periodic table in chemistry the organized array of all the chemical elements in order of increasing atomic number. There are 4 elements in group 4 in periodic table. The group number in the periodic table represents number of valence electrons of the elements in a certain group.
In this way what does group number in periodic table mean. Finally IUPAC assigns collective names lanthanoids and actinoids and group numbering 1 to 18 and has investigated the membership of the group 3 elements. Group 0 is sometimes called Group 8.
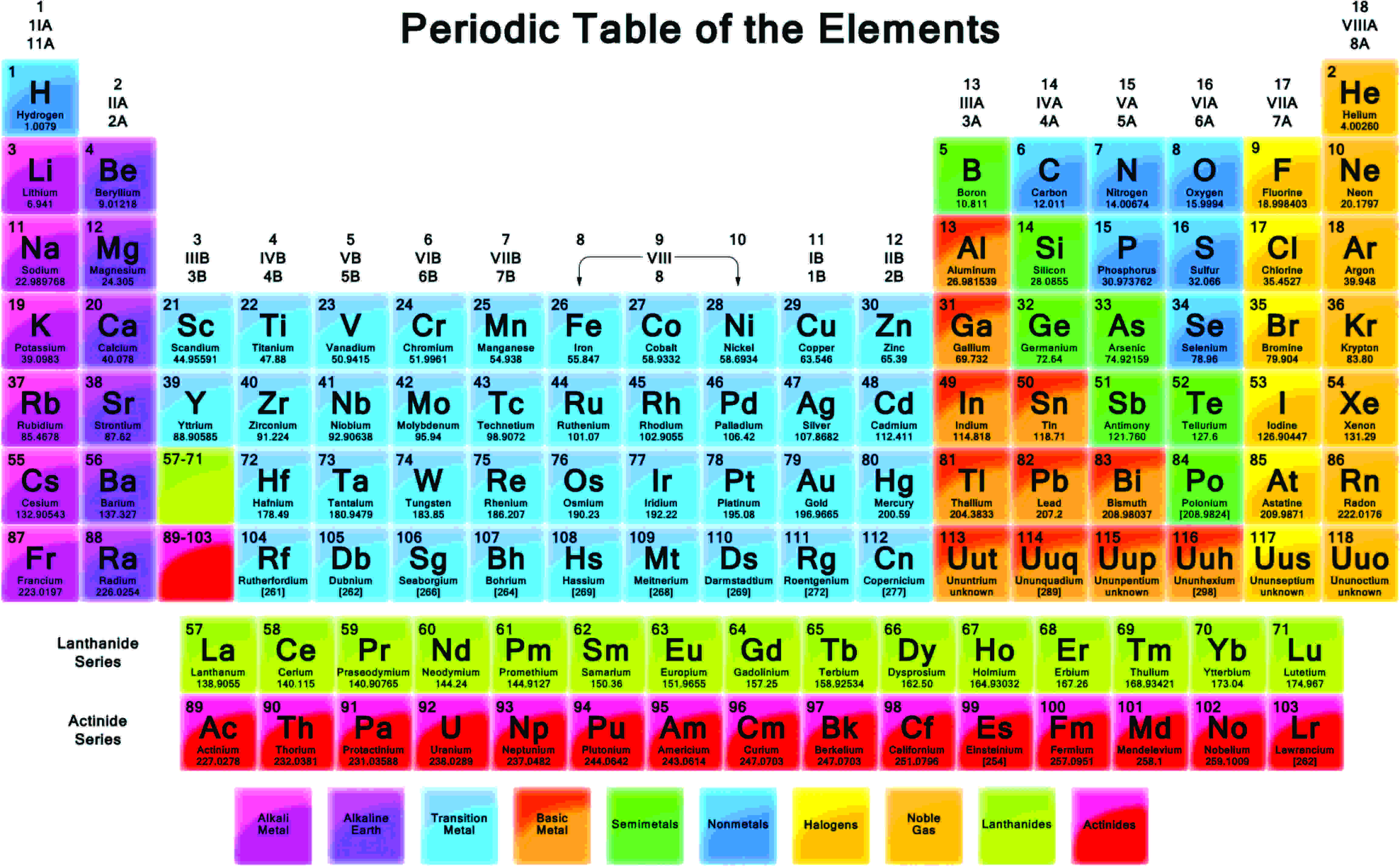
The Periodic Table has 118 elements which organized on the basis of atomic number and grouped based on similarity in chemical properties. See About the Periodic Table for information on how Group can be used to characterize an element. Periods 6 and 7 have 32 elements because the two bottom rows that are separated from the rest of the table belong to those periods.
Values in italics represent theoretical or unconfirmed oxidation numbers. PubChem is working with IUPAC to help make information about the elements and the periodic table machine-readable. Rows in the periodic table.
This number is equal to the group number. Because they have the same number of valence electrons elements in a group. Back to Elements List.
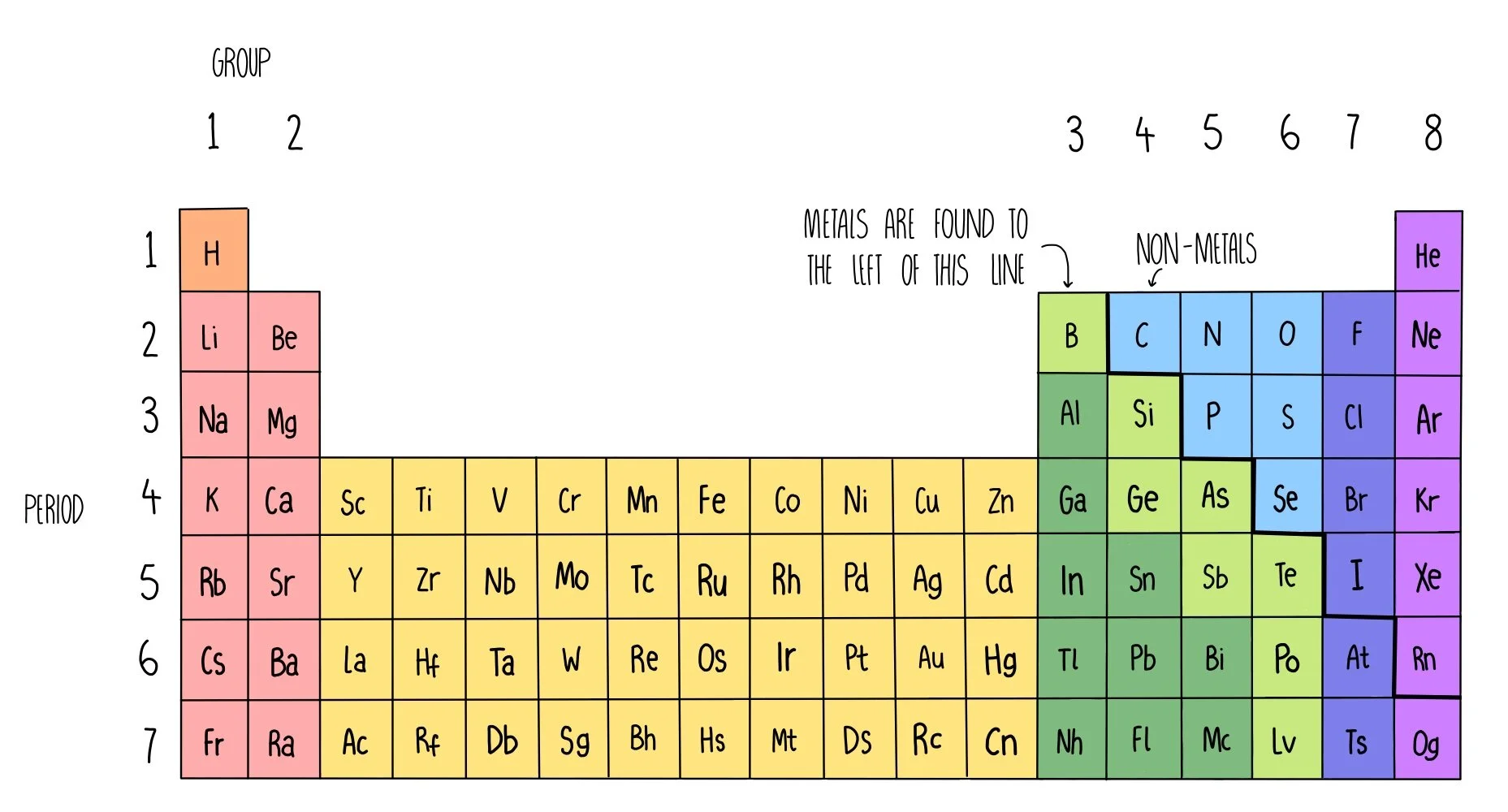
The outer electrons are called valence electrons. The notation Im using for the bit of Periodic Table shown above is. The elements are arranged according to their atomic number on the modern periodic table.
Group 0 elements helium He neon Ne argon Ar krypton Kr xenon Xe and radon Rn have full outer shells. There are only 18 groups in the periodic table that constitute the columns of the table. The main groups are numbered from 1 to 7 going from left to right and the last group on the right is Group 0 the block in between Group 2 and Group 3 is where the transition metals are placed.
The d-block elements shown in blue. The group number is an identifier used to describe the column of the standard. When the elements are thus arranged there is a recurring pattern called the periodic law in their properties in which elements in the same column group have similar properties.
The group number in the periodic table represents number of valence electrons of the elements in a certain group. Atomic Number Group 4 Elements in Periodic Table. In this system the main groups shown in red and green are numbered from 1 to 8 or from 1 to 7 and then 0 - the noble gases are sometimes described as Group 8 sometimes as Group 0.
The f-block columns between groups 3 and 4 are not numbered. Click to see full answer. In the periodic table the vertical columns are called groups and the horizontal rows are.
Period 1 has only two elements hydrogen and helium while periods 2 and 3 have 8 elements. Bold numbers represent the more common oxidation states. Groups Elements in the same group have the same number of electrons in their outermost energy level.
Alkali metals or lithium family. Alkaline earth metals or beryllium family. The elements which are in the same groups have the same number of electrons in outermost orbit ie 1st group has 1 electron in outermost orbit 2nd has 2 electrons in outermost orbit 13th group has 3 14th has 4 and so on.
Many periodic tables list numbers for element groups which are columns of the periodic table. 2 rijen Group numbers. For example all the elements.
This periodic table contains the oxidation numbers of the elements. This table also contains the element number element symbol element name and atomic weights of each element. This is also the system used by current UK examiners.
There are 18 numbered groups in the periodic table. All chemical elements have unique atomic numbers or Z. The first element is Hydrogen H with atomic number 1 and the last element is Oganesson Og with atomic number 118.
The elements in a group share the same number of valence electrons and thus have many common chemical and physical properties. Lanthanoids and Actinoids are numbered as 101 and 102 to separate them in sorting by group.