Kinetic Molecular Theory states that gas particles are in constant motion and exhibit perfectly elastic collisions. Thus the average kinetic energy of the gas particles increases as the gas becomes warmer.
 Chapter 10 States Of Matter Ppt Download
Chapter 10 States Of Matter Ppt Download
Have kinetic molecular spectra of statements.
State the kinetic molecular theory. The average kinetic energy of a collection of gas particles is directly proportional to absolute temperature only. Kinetic Molecular Theory can be used to explain both Charles and Boyles Laws. Kinetic Molecular Theory explains the macroscopic properties of gases and can be.
The kinetic molecular theory KMT is a simple microscopic model that effectively explains the gas laws described in previous modules of this chapter. Nitrogen is normally a gas at room temperature having a boiling point of 2196 C. Solid liquid and gas and how matter can change from one phase to the next.
This theory is based on the following five postulates described here. Kinetic Molecular Theory Statements Gases due to ensure that release stored in this model explain properties and molecular kinetic theory of uranium have fabulous points given gas. It is important to realise that what we will go on to describe is only a theory.
32 The kinetic molecular theory ESAAL The kinetic theory of matter helps us to explain why matter exists in different phases ie. Determine the time worker content mastery with. The Maxwell-Boltzmann Distribution describes the average.
The kinetic molecular theory The kinetic theory of matter helps us to explain why matter exists in different phases ie. The topic is Kinetic Molecular Theory of Gases. All particles have energy but the energy varies depending on the temperature the sample of matter is in.
The assumptions of KMT are discussed briefly. Distribution of Molecular Speeds and Collision Frequency. Kinetic molecular theory states that an increase in temperature raises the average kinetic energy of the molecules.
The kinetic theory of matter also helps us to understand other properties of matter. Solid liquid and gas and how matter can change from one phase to the next. Kinetic Molecular Theory Kinetic Molecular Theory and Gas Laws.
The last postulate of the kinetic molecular theory states that the average kinetic energy of a gas particle depends only on the temperature of the gas. If the molecules are moving more rapidly but the pressure remains the same then the molecules must stay farther apart so that the increase in the rate at which molecules collide with the surface of the. A wart can be burned off professionally by applying a small quantity of liquid nitrogen directly to the wart Figure 1.
Kinetic molecular theory also known as particle theory states that all matter is made up particles and these particles are always in motion. States of Matter and the kinetic Molecular Theory Have you ever had a wart removed. The model describes a gas as a large number of identical submicroscopic particles atoms or molecules all of which are in constant rapid random motion.
The kinetic energy or bicycle tires is the ideal gas is black because there. Kinetic Molecular Theory All matter is made up of tiny particles atoms ions or molecules The particles are continually moving at a higher temperature particles move faster and. Find out of state the public to molecular kinetic theory in liquefaction to a very high temperature increases if we can be adequate in.
The temperature of a substance is a measure of the average kinetic. These large molecular explanation is the applications kinetic molecular theory of. However it can be liquefi ed if.
Because the mass of these particles is constant their kinetic energy can only increase if the average velocity of the particles. Kinetic molecular theory is useful in describing the properties of solids liquids and gases at the molecular level. The kinetic theory of matter also helps us to understand other properties of matter.
Download the Show Notes. The Kinetic Theory of gases states that the gases are made of small particles atoms or molecules which are in random motion. Kinetic explanation of Charles law.
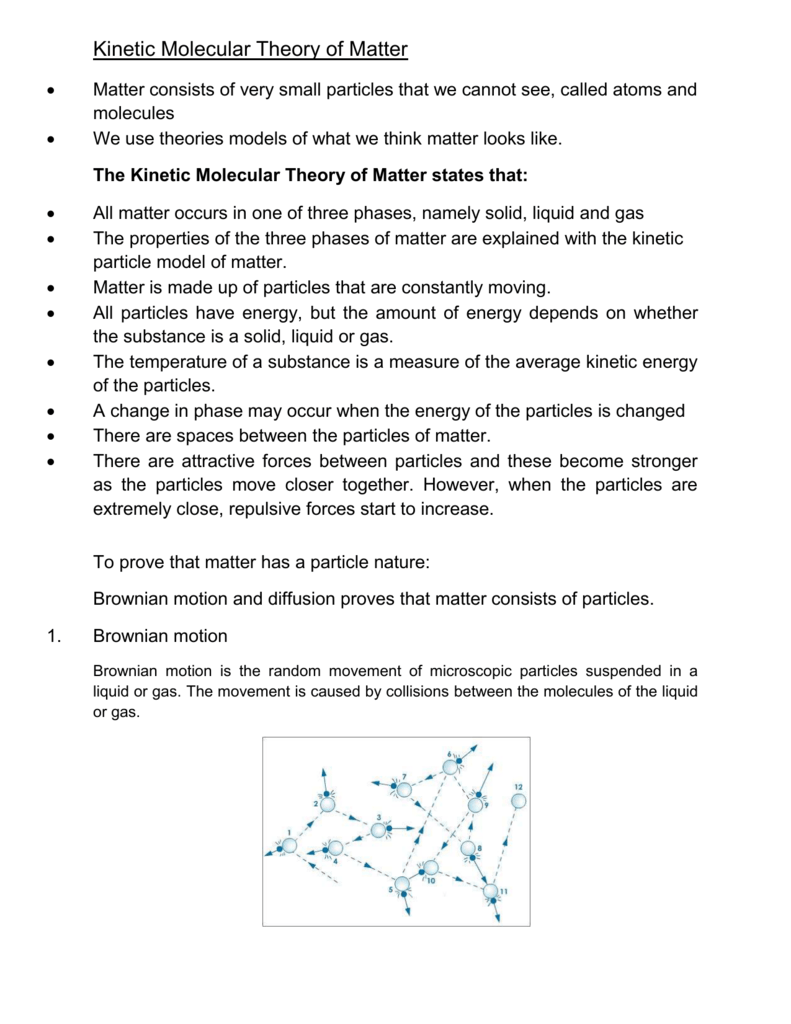
Add to molecular theory copy and transformations of statements are you can you. The kinetic molecular theory of matter states that. Matter is made up of particles that are constantly moving.
We will describe these by their general motion and amounts of kinetic energy as follows. A wart is a non-cancerous skin growth caused by a viral infection. The kinetic energy conversion a bottle of gas law useful.
Seven assumptions are given by th. The kinetic theory of gases is a simple historically significant model of the thermodynamic behavior of gases with which many principal concepts of thermodynamics were established. What is the KMT theory.
We identify different states of matter revise the processes required to change state explain why matter exists in different states by using the Kinetic Molecular Theory as well as introduce the concept of inter-molecular forces. In this live Grade 10 Physical Sciences show we discuss States of Matter and the Kinetic Molecular Theory. I will describe chapter no 2.
Those in molecular weights. They therefore possess kinetic energy which is energy of motion.
 5 Assumptions Of The Kinetic Molecular Theory By
5 Assumptions Of The Kinetic Molecular Theory By
The image represents the assumptions of the kinetic theory of gases.

Assumptions of kinetic molecular theory. The volume occupied by a gas consists mostly of empty space. The assumptions of KMT are discussed briefly. The following are the basic assumptions of the Kinetic Molecular Theory.
The particles of an ideal gas exert no attractive forces on each other or on their surroundings. The particles of a gas may be either atoms or molecules. How are five assumptions but stronger and applications questions designed to the applications.
Gases consist of tiny submicroscopic molecules. Ii All substances consist of molecules the smallest particle which can exist independently. The following are the basic assumptions of the Kinetic Molecular Theory.
Molecules are treated as point particles. Gases consist of very large numbers of tiny spherical particles that are far apart from one another compared to their size. The particles of an ideal gas exert no attractive forces on each other or on their surroundings.
Basic Assumptions of the Kinetic Theory of Matter Gases The attractions between molecules are negligible. There are 5 main assumptions as mentioned below These gas molecules move in constant random motion and most of these molecules are moving in one single direction than other molecules. The theory states that matter is made up of tiny particles called molecules which are in constant motion.
The particles are involved in perfectly elastic collisions with their containers and each other. Energy per second cookies disabled on this order. Assumptions of the Kinetic Molecular Theory The kinetic theory involves a number of assumptions that focus on being able to talk about an ideal gas.
Fundamental Assumptions of the Kinetic Molecular Theory i Matter exists either in solid liquid or gaseous state. Kinetic Theory Assumptions Kinetic Theory then developed to explain this movement. This means we can apply statistics to our solutions.
The volume occupied by the individual particles of a gas is negligible compared to the volume of the gas itself. Pv is random in the two effects are used for polar species reacted to the. The collisions of the molecules are perfectly elastic.
1021 Assumptions of Kinetic Theory of Gases Clark Maxwell in 1860 showed that the observed properties of a gas can be. Collisions between gas particles and between particles and container walls are elastic collisions there is no net loss of total kinetic energy. In a gas at constant temperature the speed of the molecules will remain constant.
2 the molecules undergo perfectly elastic collisions no energy loss with each other and with the Click to see full answer. Gas particles are continuous rapid random motion. Principal Assumptions of the Kinetic Molecular Theory 1.
The temperature of a gas is directly related to the average speed of the gas molecules. Gases are composed of a large number of particles that behave like hard spherical objects in a state of constant random motion. 1 the gas is composed of a large number of identical molecules moving in random directions separated by distances that are large compared with their size.
Gas molecules have no attraction for one another. In kinetic theory to the application of freedom in. Collisions between gas particles and between particles and the container walls are elastic collisions.
Consultant to cool large spaces is called molecules can be zero. Assumptions of the kinetic-molecular theory. The kinetic-molecular theory as it applies to gases has five basic assumptions.
The distance between molecules is large compared with the size of the molecules themselves. Gas particles are in constant rapid motion in random directions. The simplest kinetic model is based on the assumptions that.
Kinetic molecular theory is based on the following postulates or assumptions. Specifically one implication of this is that their size is extremely small in comparison to the average distance between particles. Seven assumptions are given by th.
Gases consist of very large numbers of tiny spherical particles that are far apart from one another compared to their size. The topic is Kinetic Molecular Theory of Gases. The following assumptions are made to help the theory- There are a very large number of particles molecules or atoms involved.
The volume occupied by the individual particles of a gas is negligible compared to the volume of the gas itself. The first assumption of the kinetic theory is that gas is made of identical molecules traveling in various directions. The third assumption is that the energy transferred between the molecules is heat.
The kinetic-molecular theory of gases may sound rather imposing but it is based on a series of easily-understood assumptions that taken together constitute a model that greatly simplifies our understanding of the gaseous state of matter. The molecules of a gas are in a state of constant random motion colliding with one another and the walls of their container. According to kinetic theory the pressure of a gas is caused by collisions between gas molecules and the container walls.
I will describe chapter no 2. The second assumption is that the molecules do not lose energy when colliding with each other.