A Horizontal Row Of Elements In The Periodic Table Is Called N. Get Instant Solutions 24x7.

The horizontal rows in a periodic table are called group.

Horizontal row in the periodic table. Across a period the electropositive character decreases and down a. A one or two letter representation of an element. Menedeleev arranged the elements in the periodic table in what order.
A new period begins when a new principal energy level begins filling with electrons. Members of a group typically have similar properties and electron configurations in their outer shell. Rows six and seven contain elements called transition elements which are subdivided into the.
In the periodic table which of the following identifies a horizontal row. What do elements in the same column in the periodic table have in common. In terms of electronic structure of the atom a period constitutes a series of elements whose atoms have the same number of electron shells ie.
W period x group y family z series. A vertical column in the periodic table. The vertical columns of elements are called groups or families.
Metals 5transition metals6atomic radius7. Horizontal row in the periodic table____ 2. Each row of elements on the periodic table is called a.
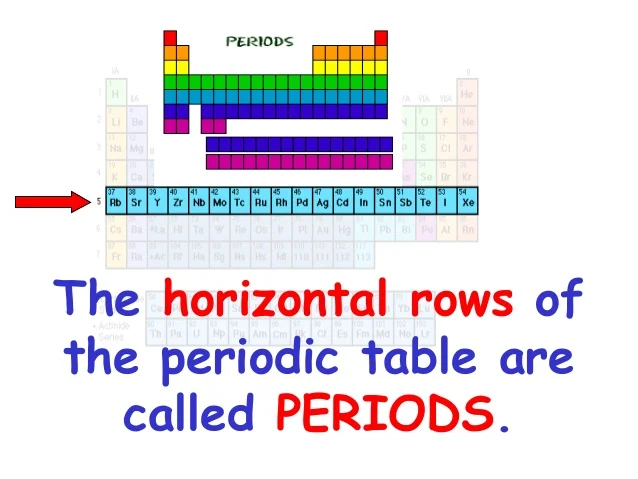
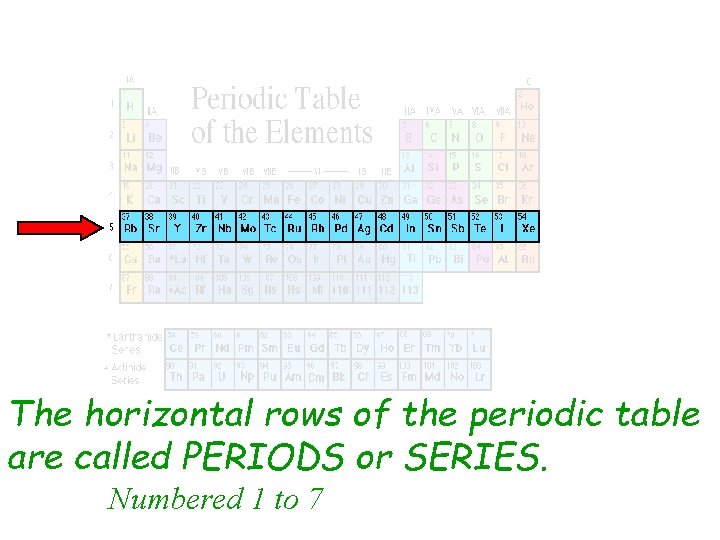
Periods are long 5 6 7 horizontal rows of elements in the periodic table and an element with three electron shells and two electrons in its valence shell belongs to period 3 6 3 1 and group 2 3 6 2. Each element bonds with the element before it. In the periodic table of elements there are seven horizontal rows of elements called periods.
Each horizontal row is called a period whereas each vertical column is called a group. On the left-hand side of the periodic table the row numbers are given as one through seven. The atomic number of each element increases by one reading from left to right.
A period is a horizontal row of the periodic table. The Horizontal Rows Of Elements In Periodic Table Are Called Quizlet. Horizontal Row Of Elements In The Periodic Table Called.
Each horizontal row in the periodic table is called a. On the periodic table the seven horizontal rows are called periods. The statement is true about Periodic law4.
Thus the given statement is false. And in the same Period electrons fill the SAME valence shell. Which is true about each element in a period or horizontal row of the periodic table.
Answer verified by Toppr. Principal quantum number n. A chart of the elements showing the repeating pattern of their properties.
Elements in the same horizontal row. Vertical column of elements in the periodic table. The Horizontal Rows Of Elements In Periodic Table Are Called Brainly.
Vertical column in the periodic table____ 3. Across a period the valence electrons increase by 1 while down a subgroup they remain same. Each element has one more outer electron than the element before it.
Each element has one more isotope than the element before it. Each element has one more outer shell than the element before it. There are seven periods in the periodic table with each one beginning at the far left.
A horizontal row on the Periodic Table is called a Period. Moving across a period from left to right the atomic number of the elements increases. Although groups generally have more significant periodic trends there are regions where horizontal trends are more significant than vertical group trends such as the f-block where the lanthanides and actinides form two substantial horizontal series of elements.
Correct option is. Each successive period in the periodic table is associated with the filling of the next higher principal energy level n 1 n 2 n 3 etc. The horizontal rows in the periodic table are called periods.
Period periodic table A period is a horizontal row in the periodic table. Each horizontal row in the periodic table is called a 2 See answers cherishma72008 cherishma72008 Answer. A horizontal row of elements in the periodic table.
A horizontal row in the periodic table. A period may be defined as horizontal row in the periodic table. Upvote 15 Was this answer helpful.
Period 1 has only two elements hydrogen and helium while periods 2 and 3 have 8 elements. The vertical columns on the periodic table are called groups or families because of their similar chemical behavior.
 In The Periodic Table How Many Columns And Groups Are There Quora
In The Periodic Table How Many Columns And Groups Are There Quora
 Elements And The Periodic Table Year 8 Science
Elements And The Periodic Table Year 8 Science
 Modern Periodic Table Periods And Groups Chemistry For Non Majors
Modern Periodic Table Periods And Groups Chemistry For Non Majors
 Each Horizontal Row Is Called A Period Properties Of Elements Change Greatly A Physical Science Middle School Periodic Table Of The Elements Science Chemistry
Each Horizontal Row Is Called A Period Properties Of Elements Change Greatly A Physical Science Middle School Periodic Table Of The Elements Science Chemistry
 Why Is It Called A Periodic Table Teenage Pregnancy
Why Is It Called A Periodic Table Teenage Pregnancy
 Periodic Table Electron Configurations The Horizontal Rows Of
Periodic Table Electron Configurations The Horizontal Rows Of
 Periodic Table Ppt Video Online Download
Periodic Table Ppt Video Online Download
 The Horizontal Rows Of The Periodic Table Are Called Periods Ppt Download
The Horizontal Rows Of The Periodic Table Are Called Periods Ppt Download