To explain why water boils at 90 o C in the mountains and 120 o C in a pressure cooker even though the normal boiling point of water is 100 o C we have to understand why a liquid boils. Advantages of Using Celsius Rather than Fahrenheit.
 Melting Point Freezing Point Boiling Point
Melting Point Freezing Point Boiling Point
Therefore the boiling point.
Freezing and boiling points of water. This Demonstration shows the boiling temperature of water on six planets all of which have different atmospheric pressures. At at high altitudes the lower pressure makes the boiling point several degrees lower. Freezing point definition is - the temperature at which a liquid solidifies.
About Press Copyright Contact us Creators Advertise Developers Terms Privacy Policy Safety How YouTube works Test new features Press Copyright Contact us Creators. Because the freezing point of pure water is 0C the sucrose solution freezes at 068C. Boiling water is no big deal to us grown-ups because we see it almost every time we cook.
So 100ºC is now the boiling point of water while 0ºC is the freezing point of water. The Celsius scale sets the freezing point and boiling point of water at 0C and 100C respectively. The red line shows the atmospheric pressure of the selected planet.
What temperature does water boil at 10000. The Fahrenheit scale defines the freezing point of water as 32F and the boiling point as 212F. The boiling point is the temperature at which the vapor pressure is equal to the atmospheric pressure.
Temperature is often measured in one of two scales. A similar property of solutions is boiling point elevation. By definition a liquid boils when the vapor.
Impurities in the water such as sea salt pressure and altitude will raise or lower the freezing point. Click to see full answer. A Fahrenheit thermometer will measure the same events at 212 for the boiling point of water and 32 as its freezing point.
The blue line shows the vapor pressure of water as a function of temperature. The change in the boiling point is calculated from. Each fifth grader sat quietly observing the water begin to heat up and.
Write a program that asks the user to enter a temperature and then shows all the substances that will freeze at that temperature and all that will boil at that temperature. A solution boils at a slightly higher temperature than the pure solvent. The boiling point elevation is the amount that the boiling point temperature increases compared to the original solvent.
A mathematical equation is used to calculate the boiling point elevation or the freezing point depression. A Celsius thermometer will measure the boiling point of water at 100 and its freezing point at 0. For example if the user enters 20 the program should report that water will freeze and oxygen will boil at that.
For pure water the boiling point is 100 degrees Celsius 212 Fahrenheit at one atmosphere of pressure and the melting point is 0 degrees Celsius 32 degrees Fahrenheit at one atmosphere of pressure. For example the boiling point of pure water at 10. The following table lists the freezing and boiling points of several substances.
Sign up for the The scale places the freezing and boiling points of water exactly 180 degrees apart because in this scale the freezing point of water is 32 degrees 32 F and the boiling point is 212 degrees. Pure water has a freezing point of 32 o F 0 o C at sea level under normal pressure. The Celsius scale and the Fahrenheit scale.
There are 100 degrees between the freezing 0 and boiling points 100 of water on the. Textatm is 100texto textC while the boiling point of a 2 saltwater solution is about 102texto textC. At standard temperature refers to 0 C or 15 C at sea level Celsius is great for measuring water temperature using its 0 to 100 scalefor instance when you heat milk.
If water was very easy to freeze or boil drastic changes in the environment and so in oceans or lakes would cause all the organisms living in water to die. Calculate the freezing point and boiling point of 109 g of FeCl3 in 130 g of water assuming complete dissociation. The boiling and freezing points of water enable the molecules to be very slow to boil or freeze this is important to the ecosystems living in water.
I cant tell you how surprised I was the first time I boiled water for my fifth graders. How to use freezing point in a sentence. In a typical pressure cooker water can remain a liquid at temperatures as high as 120 o C and food cooks in as little as one-third the normal time.
This week we will be identifying the boiling point of water and the freezing melting point of water. In other words it was upside-down.
 Boiling Point Freezing Melting Point Anchorchart Teaching Science Science Lessons Science Anchor Charts
Boiling Point Freezing Melting Point Anchorchart Teaching Science Science Lessons Science Anchor Charts
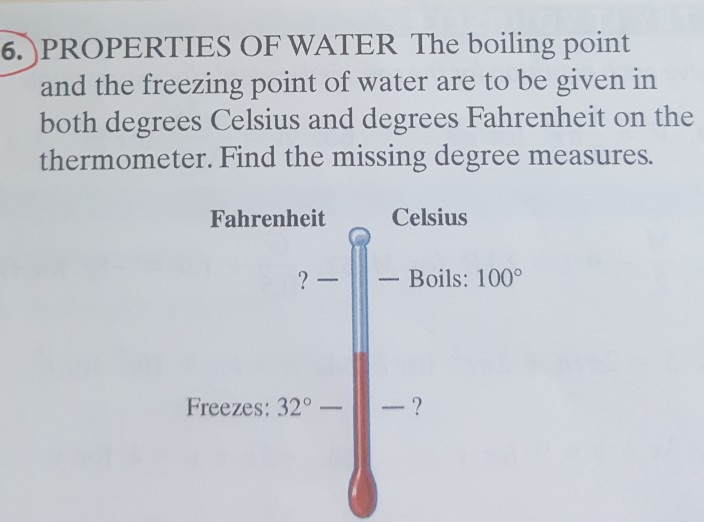 Solved 6 Properties Of Water The Boiling Point And The F Chegg Com
Solved 6 Properties Of Water The Boiling Point And The F Chegg Com
 What Is Water S Boiling Point In Fahrenheit The Millennial Mirror
What Is Water S Boiling Point In Fahrenheit The Millennial Mirror
/the-freezing-point-of-water-609418_FINAL-01f50f5f4f7d4a39854bebcc59df1aa4.gif) What Is The Freezing Point Of Water
What Is The Freezing Point Of Water
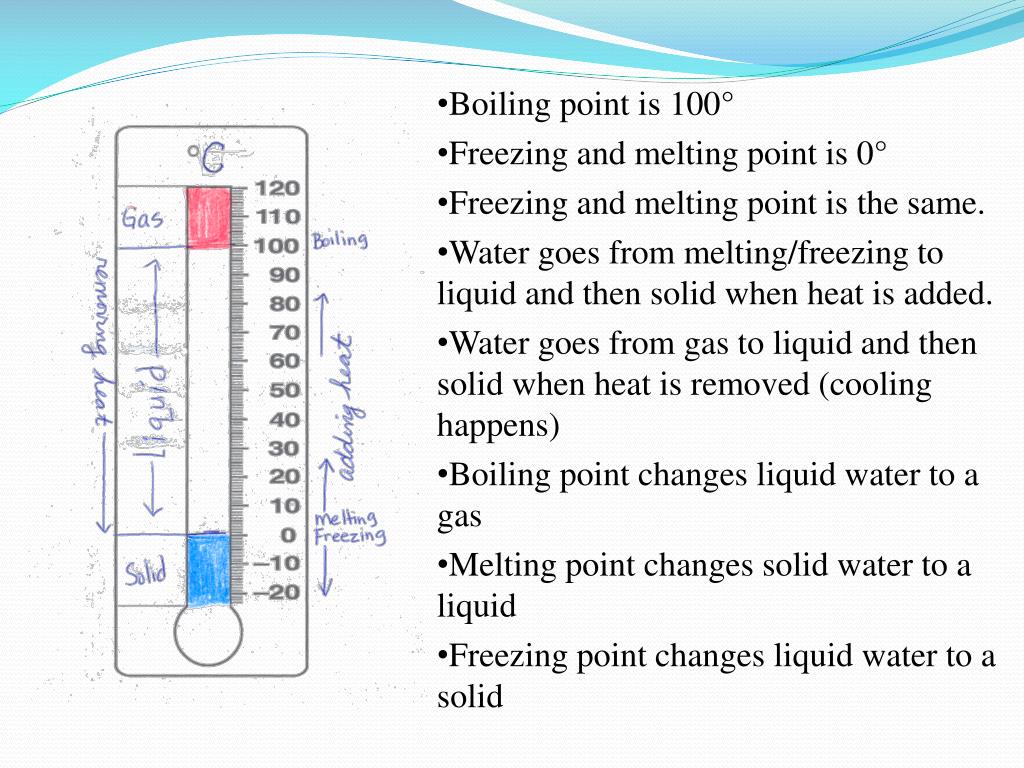
 Ppt Physical Properties Of Water Boiling Point Melting Point And Freezing Point Powerpoint Presentation Id 3245093
Ppt Physical Properties Of Water Boiling Point Melting Point And Freezing Point Powerpoint Presentation Id 3245093
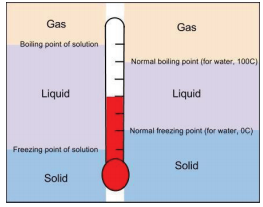 13 9 Freezing Point Depression And Boiling Point Elevation Making Water Freeze Colder And Boil Hotter Chemistry Libretexts
13 9 Freezing Point Depression And Boiling Point Elevation Making Water Freeze Colder And Boil Hotter Chemistry Libretexts
What Is The Freezing Point Of Iron In Celsius
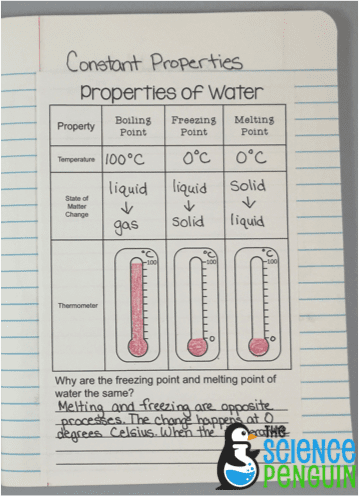 Constant Properties Of Water Boiling Point Melting Point And Freezing Point The Science Penguin
Constant Properties Of Water Boiling Point Melting Point And Freezing Point The Science Penguin
